A set of empirical rules used to determine whether an ionic compound is soluble. Determine which ionic compound is dissolved in solution.
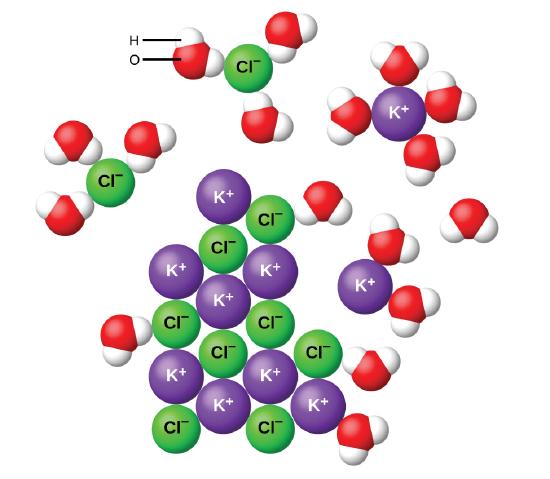
7 5 Aqueous Solutions And Solubility Compounds Dissolved In Water Chemistry Libretexts
With ionic compounds chemists often talk about ionic concentrations or the concentrations of the.
. When you prepare a molar solution avoid this problem by adding solvent to your solute to reach a specific volume. Determine which ionic compound is dissolved in solution. Therefore a solution that conducts electricity well.
This means that when one mole of salt NaCl is dissolved in water the resulting solution does not contain any molecules of NaCl it contains the ions Na and Cl -. In this case you know that one formula unit of sodium chloride NaCl contains one sodium cation Na and one. Specifically we are going to work with ionic compounds that are soluble in water dissocia.
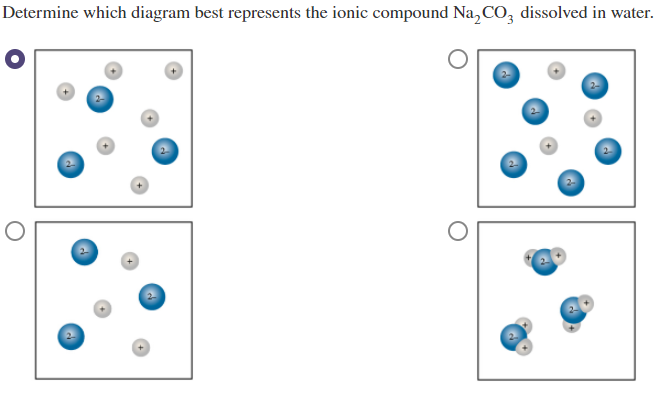
A homogeneous mixtures of a substance with water. Consider the diagram of an aqueous solution of a soluble ionic compound Determine which ionic compound is dissolved in solution. Consider the diagram of an aqueous solution of a soluble ionic compound Determine which ionic compound is dissolved in solution.
Consider the diagram of an aqueous solution of a soluble ionic compound. Experts are tested by Chegg as specialists in their subject area. One end has a positive charge while the other has a negative.
What determines conductivity of a solution. Answer to Solved Consider the diagram of an aqueous solution of a. Determine which ionic compound is dissolved in solution.
Th is is possible because both solute and solvent consist of non-polar molecules. Naci MgBry AICI Na PO bout Cine. 2 2 2 Determine which ionic compound is dissolved in solution.
Determine whether each compound is soluble or insoluble. Consider the diagram of an aqueous solution of a soluble ionic compound. These are for common cations too long for me to type out.
Oil layer water Figure 7 a Oil and water are immiscible liquids. 100 3 ratings Transcribed image text. The concentrations of ions the type of ions and the temperature of the solution.
Chemistry questions and answers. Naci MgBry AICI Na PO bout Cine cy help. In this episode of Real Chemistry you will learn how to determine if an ionic compound is soluble or insoluble.
There are three main factors that affect the conductivity of a solution. Ionic compounds dissociate completely when dissolved. All nitrates are soluble.
A solution containing a solute that dissociates into ions. Chemistry questions and answers. Consider the diagram of an aqueous solution of a soluble ionic compound.
Now determine moles per liter of solution. One of my favorite chemistry books General Chemistry by Linus Pauling has some very nice rules of thumb for predicting solubility on page 453 Dover edition. 0910 moles Na Youre dealing with a soluble ionic compound so you know for a fact that it dissociates completely in aqueous solution to produce cations which are positively charged ions and anions which are negatively charged ions.
M 062 moles NaCl 050 liter solution 12 M solution 12 molar solution Note that I assumed dissolving the 6 grams of salt did not appreciably affect the volume of the solution. Up to 24 cash back dissolves in hydrocarbon solvents to give a purple solution Figure 9a. In this video we will go through how to find ions in a compound.
If a compound does conduct electricity when dissolved in solution or molten it is an ionic compound. This worked example problem illustrates the steps necessary to calculate the concentration of ions in an aqueous solution in terms of molarityMolarity is one of the most common units of concentration. Some ionic compounds arent stuck together very.
London dispersion forces loosely attract the iodine molecules to the hydrocarbon allowing iodine to dissolve. Water molecules H 2 O have an unusual structure which makes them similar to a magnet. When you drop an ionic compound in water these water magnets will gather around it trying to pull the positive and negative ions apart.
Na SO MgCl2 OKCIO O Ca NO32. 2 OCaCl ОКСО O MgSO4. We review their content and use your feedback to keep the quality high.
Consider the diagram of an aqueous solution of a soluble ionic compound. Answer 1 of 4.

Aleks Calculating The Solubility Of An Ionic Compound When A Common Ion Is Present Youtube
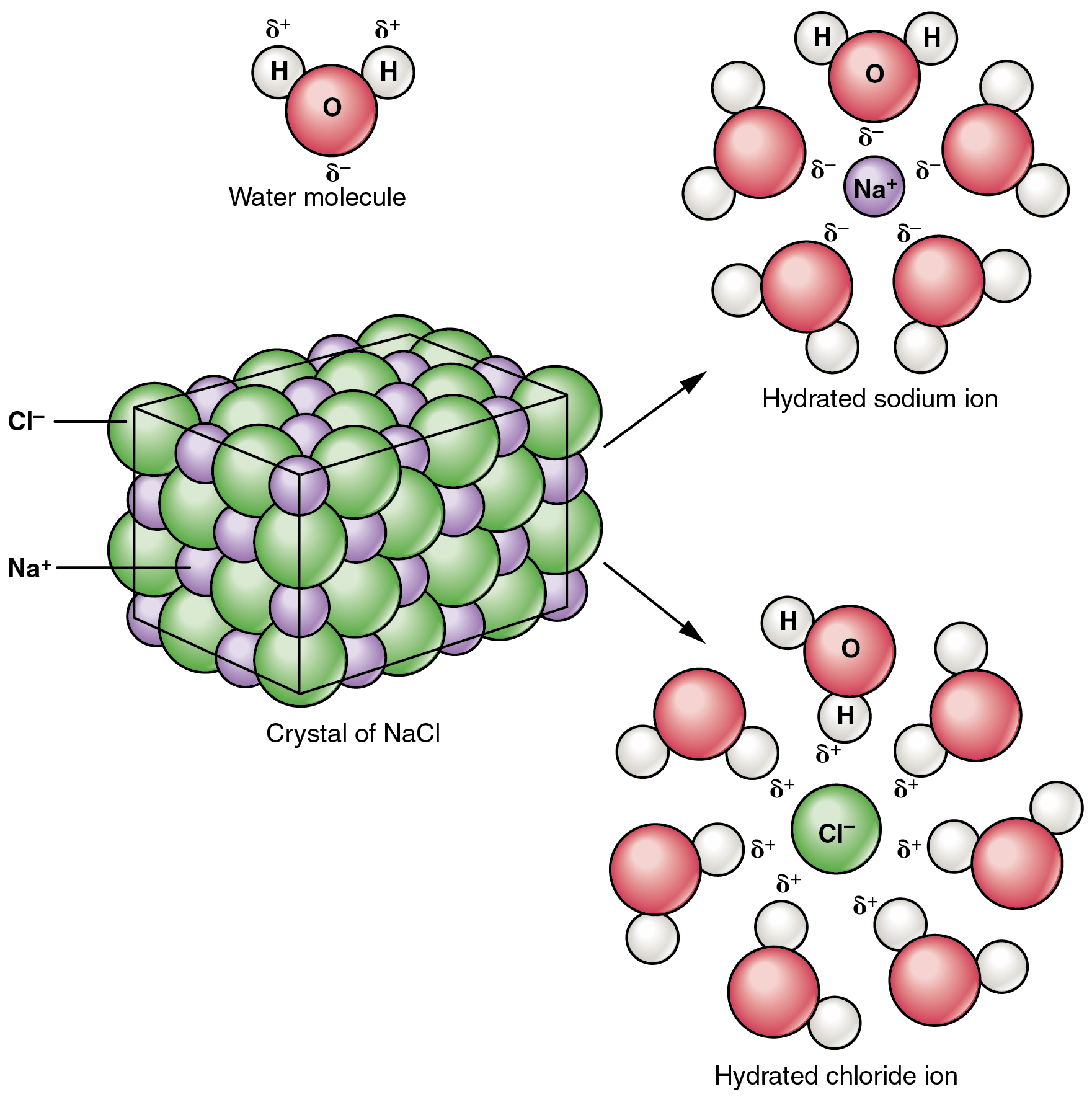
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy

Solved Determine Which Diagram Best Represents The Ionic Chegg Com

0 Comments